| (Image of Pregabalin Medication Label) |
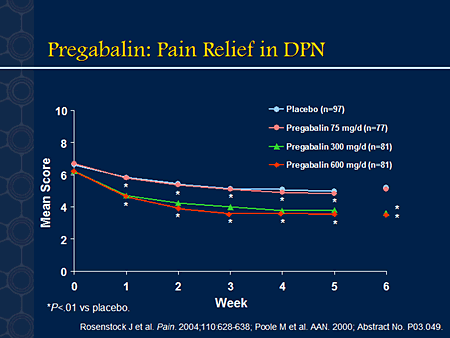
Pregabalin, also known as Lyrica, was first introduced by Pfizer Inc. in 2004 as treatment for neuropathic pain with associated diabetic peripheral neuropathy. In the year to follow, Pregabalin was then FDA approved as treatment for partial epileptic seizures. Later, in 2007, Pregabalin was declared the first approved medication to treat fibromyalgia. However, Pregabalin is regulated under the Controlled Substances Act and is classified as a Schedule V drug by the Drug Enforcement Agency (DEA).
Generic Name:
|
Pregabalin
|
|
Trade Name:
|
Lyrica
|
|
Chemical
Name:
|
(S)-3-(aminomethyl)-5-methylhexanoic
acid
|
|
Classification:
|
Anticonvulsant; Antiepileptic; Antiseizure
|
|
Company
|
Pfizer Inc.
|
|
Year
Introduced
|
2004
|
|